How Many Hydrogen Atoms Are in Ammonium Sulfate
Conversion between moles and particles. In addition ammonia emits a strong odor but ammonium does not smell at all.

How To Find The Number Of Atoms In Nh4 2so4 Ammonium Sulfate Youtube
Ammonium sulfide Hydrogen bromide Hydrogen sulfide Ammonium bromide Oxidation Numbers 41.

. K potassium. Water electrolysis is a quite old technology started around two centuries back but promising technology for hydrogen production. Ammonium nitrate --- water and dinitrogen oxide laughing gas.
Hydrogen sulfate or bisulfate HSO 4-hydrogen sulfite or bisulfite HSO 3-hydrogen phosphate HPO 4 2-hydroxide OH-hypochlorite ClO-nitrate NO 3-nitrite NO 2-oxalate C 2 O 4 2-perchlorate ClO 4-permanganate MnO 4-peroxide O 2 2-phosphate PO 4 3-phosphite PO 3 3-silicate SiO 3 2-sulfate SO 4 2-sulfite SO 3 2-thiocyanate SCN-thiosulfate S 2 O 3 2-. Convert grams NH42SO4 to moles or moles NH42SO4 to grams. Many atoms of each element there are in a formula unit.
You can also cause a double replacement chemical reaction when you combine an acid and a base. How many atoms of. One sodium atom one chlorine atom in a formula unit CaCl 2.
While ammonia is a neutral non-ionized molecule weak base ammonium is an ion carrying a positive charge. With chlorine monochloramine is formed. What is the mass of 0288 moles of plumbous sulfate.
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH 4. Na sodium. Reactions that use an acid and a base as reactants is known as a.
In a formula parentheses are used around a polyatomic ion only when there are 2 or more of that polyatomic ion in a formula unit ie when the subscript is not 1. Nitrosamines contain two nitrogen atoms but neither SLS nor formaldehyde contain nitrogen atoms. Zn 2 zinc.
Here the four N-H bonds are arranged in trigonal pyramidal. 140067 10079442 32065 1599944 Percent composition by element. The hydrogen in ammonia is susceptible to replacement by a myriad of substituents.
For questions on oxidation number read the symbol x as the oxidation number of x a BrO3- Br 3O -1 Since oxygen almost always has an oxidation number of -2 we can substitute this value and solve for the oxidation number of Br. H hydrogen. We therefore need a series of rules that allow us to unambiguously name positive and negative ions before we can name the salts these ions form.
Thus hydrogen has an atomic number of 1 while iron has an atomic number of 26. Sulfates including Sodium Lauryl Sulfate SLS along with its cousins are a class of chemicals known as surfactants. Ammonium ion has a trigonal pyramidal structure.
However this group of atoms is most stable when it has either lost of gained an electron and thus existed as a charged ion. How many moles is 78 x 1025 molecules of phosphorus pentachloride. Ammonium ion is a polyatomic on.
This number of protons is so important to the identity of an atom that it is called the atomic number of the element. NH42S ammonium sulfide K3PO4 potassium phosphate ZnNO32 zinc nitrate Fe2SO43 ironIII sulfate or ferric sulfate CuCO3 copperII carbonate or cupric carbonate Note. The IUPAC name of ammonium ion is azanium.
When dry ammonia gas is heated with metallic sodium it converts to sodamide NaNH 2. The major factor that determines the proportion of ammonia to ammonium in water is pH. Therefore the two cannot react to form a nitrogen-containing nitrosamine.
Ammonium ion is an inorganic ion composed of one nitrogen atom and four hydrogen atoms. Pentavalent ammonia is known as λ 5-amine or more commonly ammonium hydride. How many grams are in 63 moles of ammonium chloride.
How many molecules are in 155 moles of hydrogen gas H2. These polyatomic ions are extremely common in chemistry and thus it is important to be able to both recognize and name them. All atoms of iron have 26 protons in the nucleus.
How many moles are in 245 g of hydrogen gas H2. Silver nitrate sodium chloride --- silver chloride and sodium nitrate. They are detergents originally developed as degreasers to clean carpets engines laundry etc.
Sodium Lauryl Sulfate Sodium Laureth Sulfate and Ammonium Lauryl Sulfate are the three most common Sulfates used in personal care products. Ca 2 calcium. One calcium atom two chlorine atoms in a formula unit Mg 3N 2.
The molar mass of this cation is 18039 gmol. Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations NR 4 where one or more hydrogen atoms are replaced by organic groups indicated by R. All atoms of hydrogen have one and only one proton in the nucleus.
It has four N-H bonds. This compound is also known as Ammonium Sulfate. Monatomic positive ions have the name of the element from which they are formed.
Three magnesium atoms two nitrogen atoms in a formula unit The presence of a metal in a chemical formula indicates an ionic compound which is composed of positive ions cations. A student is using colored beads to make a model of aluminum sulfate Al 2 SO 4 3Aluminum atoms are represented by blue beads sulfur atoms by. Ammonium is a compound containing one nitrogen and four hydrogen atoms NH 4.
This crystalline solid is only stable under high pressure and decomposes. Although nitrosamines have been associated with several types of cancer and many are classified by IARC as known possible or probable carcinogens depending on the chemical species. Sulfuric acid barium hydroxide --- barium sulfate and water.
While there are many such ions in the world you are responsible for knowing the ions listed in the following tables. This work reviewed the. Each element has its own characteristic atomic.
It is formed by the protonation of ammonia NH 3.

Show Details For Ammonium Sulfate

2 Exp Ammonium Iron Ii Sulfate Standard Solution Titration With Potassium Permanganate Youtube

Equation For Nh4 2so4 H2o Ammonium Sulfate Water Youtube

How To Find The Number Of Atoms In Nh4 2so4 Ammonium Sulfate Youtube

So42 Lewis Structure Sulphate Ion How To Find Out Lewis Molecules

What Is Ammonium Sulfate Or Nh4 2so4 Ammonium Carbonate Solubility Ammonium Nitrate
Cerium Iv Ammonium Sulfate Dihydrate Ceh20n4o18s4 Pubchem

Calculate The Number Of Hydrogen Atoms In 39 6 G Of Ammonium Sulfate Nh4 2so4 Brainly In

Polyatomic Ions In A Different Language But Thankfully Science Is Universal Pingram Bilder Fur Sie Teaching Chemistry Chemistry Education Organic Chemistry

43086 58 4 Ammonium 15n Sulfate Bis Ammonium 15n Sulfate Ammonium Sulfate 15nh4 2so4 Ammonium 15n2 Sulfate H No S Trc
Ammonium Sulfate Hydrate H10n2o5s Pubchem
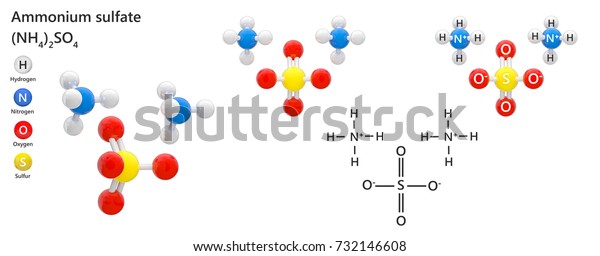
Ammonium Sulfate Formula Nh42so4 Inorganic Salt Stock Illustration 732146608 Shutterstock

Ammonium Sulfate Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic

Calculate The Number Of H Atoms In 39 6g Of Ammonium Sulphate Nh 4 2 So 4 Youtube

Ammonium Sulfate Nh4 2so4 Structure Molecular Mass Preparation Properties Uses And Faqs Of Ammonium Sulfate
How Many Hydrogen Atoms Are There In The Formula For Ammonium Sulfate Quora
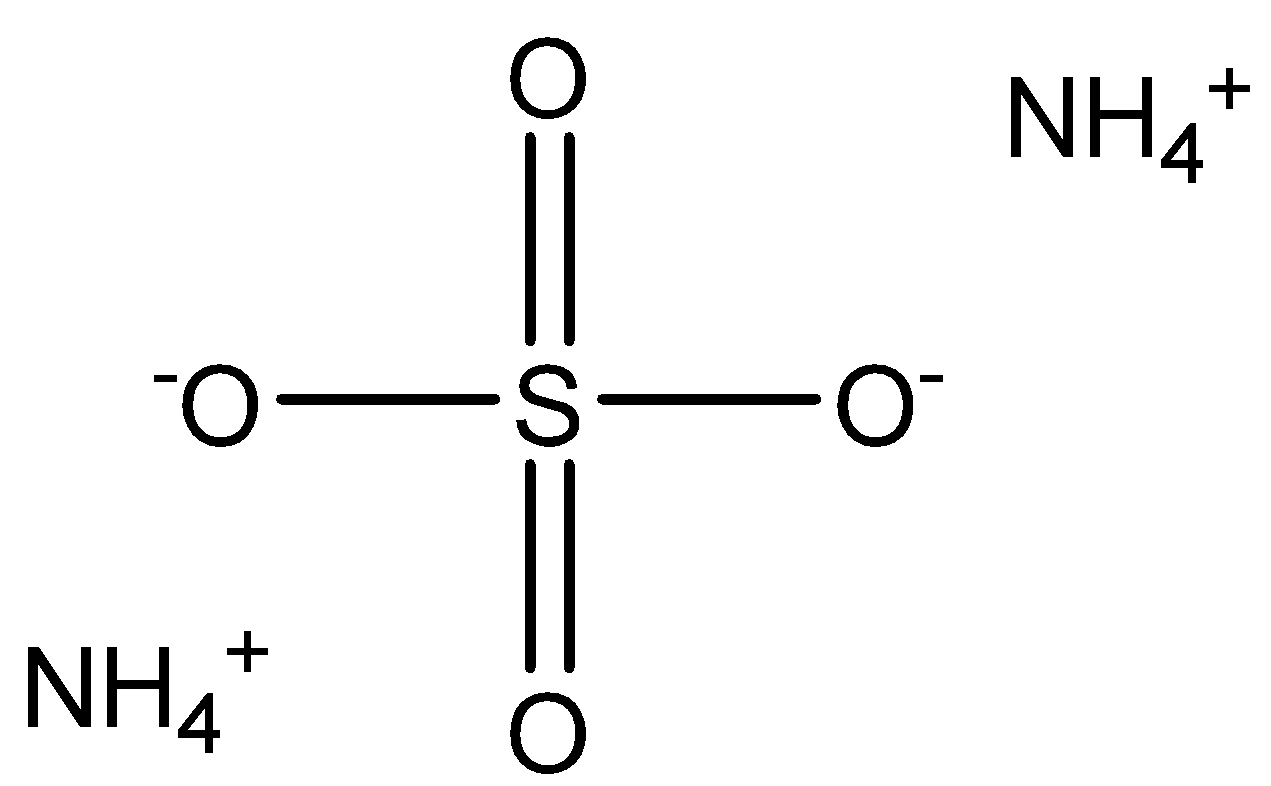
What Is The Molar Mass Of Ammonium Sulfate Class 12 Chemistry Cbse


Comments
Post a Comment